
Contact
1101 Chevron219 Parkman Avenue
Pittsburgh, PA 15260
412-624-8240
My Website >
Research Overview
Organic synthesis and medicinal chemistry, radical chemistry, fluorous chemistry, ligated borane chemistry
Radical Chemistry
Over the years, we have made many contributions to synthetic radical chemistry. Highlights include our early synthesis of hirsutene, which was the first natural product to be made by a cascade radical cyclization. Later we made camptothecin and scores of analogs by cascade radical reactions. Other natural products that we have synthesized include capnellene, silphiperfolene, hypnophillin, coriolin, mappicine (and ~750 analogs) and meloscine. We have also pioneered new radical atom transfer reactions, radical translocation reactions, and stereoselective radical reactions.

Fluorous Chemistry
Our group pioneered many new techniques and reactions as the discipline of fluorous chemistry began to emerge and prosper. We introduced fluorous synthesis (fluorous tagging), fluorous triphasic reactions, light fluorous methods, phase vanishing reactions (with Ilhyong Ryu), and fluorous mixture synthesis. Our 1997 invention of fluorous solid phase extraction (FSPE) enabled much subsequent work in our group and others. Techniques of fluorous mixture synthesis produced stereoisomer libraries of about a dozen different natural products, including pheromones, mating hormones and macrolactones with anticancer and antibiotic activities.
Ligated Borane Chemistry
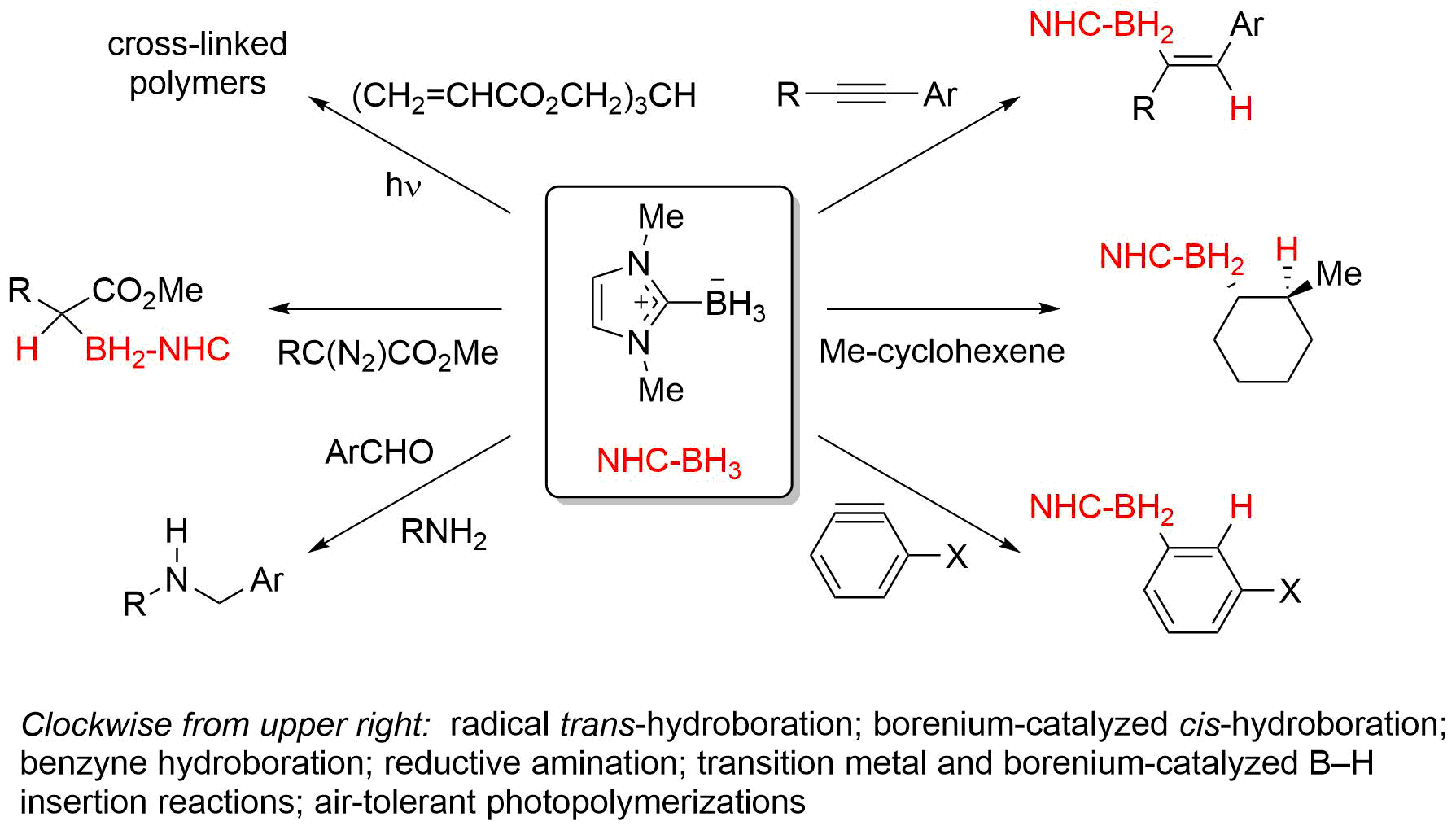
Most recently, our work has focused on synthesis and reactions of N-heterocyclic carbene boranes (NHC-boranes). NHC-boranes are easy to make, and they are typically white solids that are stable to ambient lab conditions. They are promising reagents for use is various radical, ionic and metal-catalyzed reactions.
Awards
- 2015 - Chaire Joliot, École Supérieure de Physique et de Chimie Industrielles (ESPCI), Paris
- 2014 - American Chemical Society Ernest Guenther Award in the Chemistry of Natural Products
- 2010 - Honorary Doctorate, Université Pierre et Marie Curie (UPMC), Paris; Chaire d’Excellence, Agence National de la Recherche (ANR), France
- 2009 - Fellow of the American Chemical Society; Provost’s Award for Excellence in Mentoring, University of Pittsburgh
- 2008 - American Chemical Society Award for Creative Work in Fluorine Chemistry; Chemistry; Blaise Pascal International Research Chair, Préfecture de la Région d’Ile-de-France
- 2007 - University of Pittsburgh Innovator Award; Harry and Carol Mosher Award, ACS Santa Clara Valley Section
- 2006 - The Pittsburgh Award, ACS Pittsburgh Section; Morley Medal, ACS Cleveland Section
- 2003 - Pittsburgh Magazine, Innovator of the Year Award
- 2000 - American Chemical Society Award for Creative Work in Organic Synthesis
- 1999 - Chancellor’s Distinguished Research Senior Scholar Award, University of Pittsburgh
- 1998 - Janssen Prize for Creativity in Organic Synthesis
- 1988 - American Chemical Society Cope Scholar Award