
Contact
219 Parkman Avenue
Pittsburgh, PA 15260
412-624-3058
My Website >
Research Overview
Living cells orchestrate the location and activity of millions of diverse molecules into organized nano-sized factories that power the cell. We would like to understand how to dynamically place molecules where we want within living cells and how to program cells to make complex decisions. Developing these principles into a cellular engineering code will allow us to create designer cell-based therapeutics and construct biomimetic nanomaterials to address current health challenges.
The bacterial cell as a three-dimensional chemical machine
Encoded within cells are advanced "nanotechnologies" that, when fully understood, will allow us to perform chemistry in a way Richard Feynman might have imagined - coordinating the location and timing of interconnected reactions like a nano-sized chemical factory. From stem cells to bacteria, these types of nanotechnologies drive the asymmetric distribution of proteins within a cell culminating in distinct functions and cell types upon division. Self-assembled coiled-coil scaffolding proteins represent a diverse and poorly understood set of proteins that coordinate the spatial and temporal regulation of protein networks and help push cells beyond bags of molecules. Our lab uses biophysical spectroscopy techniques, mechanistic biochemistry, and genetics to understand the design rules of how scaffolding proteins from different bacteria recruit, regulate and rewire signaling pathways. In the long-term we envision exploiting the knowledge we learn of self-assembled coiled-coil scaffolding proteins to construct biomimetic "intelligent" nanomaterials.
How do simple bacterial cells make decisions?
Bacteria must continually detect and interpret environmental as well as intracellular conditions to inform sophisticated lifestyle changes such as virulence, symbiosis, sporulation, and biofilm formation. We are interested in the molecular machinery that enables single cells to make these "intelligent" decisions. Rather than being wired together by static circuit boards, biological signaling circuits comprise dynamic small-molecule/receptor binding events, allosteric conformational switches, protein-protein interactions, and phosphorylation reactions, all logically integrated into a decision making circuit. Applying biophysical chemistry techniques, we aim to map the pattern of molecular interactions between the components that process information - from the initial signals to the final output response - by examining how the bacterium Caulobacter crescentus uses a large set of signaling and scaffolding proteins to divide asymmetrically.
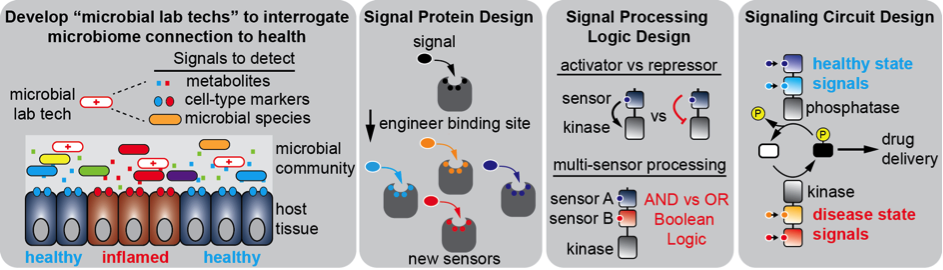
Engineering complex signaling circuits for cell-based therapeutics
Synthetic biology combined with protein engineering holds promise for broad applications of customized cells that function as therapeutics and generate biofuels. However, the early stages of development of this technology require systematic ways to safely and effectively execute cellular functions. In our lab, we are programming signaling proteins within bacteria on two discrete levels: (1) engineering sensory protein binding sites to generate designer biosensors that detect a diverse set of signals, and (2) engineering interactions and crosstalk between signaling proteins to enable advanced information processing. Development of the programming capacity of bacterial signaling systems will be used in the long-term as probiotic "microbial lab techs" for a variety of purposes, including diagnosis of digestive diseases, detection of water contamination, and coordination of metabolic processes for biofuel production.
Awards
- Jane Coffin Childs Postdoctoral Fellowship (JCCF) (2011-2014)
- Distinguished Dissertation Award Finalist, Council of Graduate Schools/Proquest (2011)
- Charles T. Lester Award, Emory University (2010)
- Microscopy Society of America (MSA) Presidential Student Award (2008)
- Achievement Awards for College Scientists (ARCS) Scholar Fellowship (2008-2009)
- HHMI ORDER (On Recent Discoveries of Emory Researchers) Teaching Scholar (2007)
- Osbourne R. Quayle Fellowship (2007)